New E-Book Targets Medical Device Market
The med tech industry in the U.S. is experiencing an upswing in revenue according to a recent study...

The med tech industry in the U.S. is experiencing an upswing in revenue according to a recent study...

If you're a regular reader, you know we've recently explored the automobile, airplane and medical...
Medical device makers have a lot to keep up with these days when it comes to measuring parts....
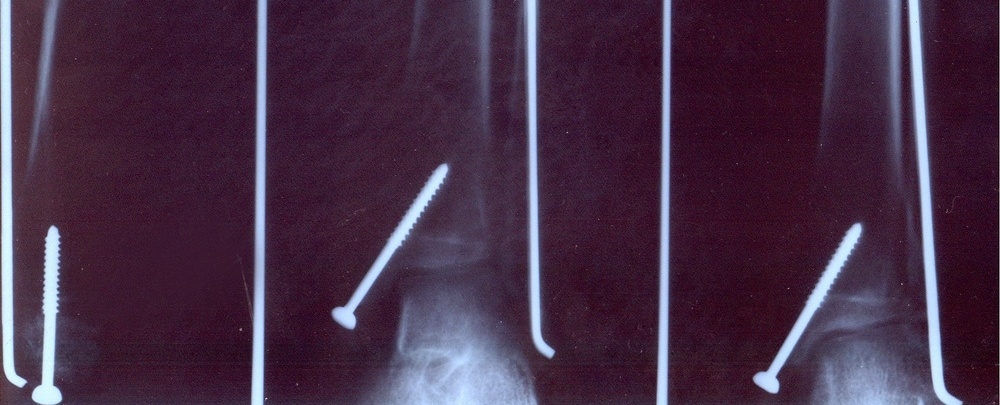
These days baby boomers are more active than ever. As 70 becomes the new 50, the demand for...

These...